-
PDF
- Split View
-
Views
-
Cite
Cite
Heiko Graf, Birgit Abler, Antonie Hartmann, Coraline D. Metzger, Martin Walter, Modulation of attention network activation under antidepressant agents in healthy subjects, International Journal of Neuropsychopharmacology, Volume 16, Issue 6, July 2013, Pages 1219–1230, https://doi.org/10.1017/S1461145712001368
Close - Share Icon Share
Abstract
While antidepressants are supposed to exert similar effects on mood and drive via various mechanisms of action, diverging effects are observed regarding side-effects and accordingly on neural correlates of motivation, emotion, reward and salient stimuli processing as a function of the drugs impact on neurotransmission. In the context of erotic stimulation, a unidirectional modulation of attentional functioning despite opposite effects on sexual arousal has been suggested for the selective serotonin reuptake-inhibitor (SSRI) paroxetine and the selective dopamine and noradrenaline reuptake-inhibitor (SDNRI) bupropion. To further elucidate the effects of antidepressant-related alterations of neural attention networks, we investigated 18 healthy males under subchronic administration (7 d) of paroxetine (20 mg), bupropion (150 mg) and placebo within a randomized placebo-controlled cross-over double-blind functional magnetic resonance imaging (fMRI) design during an established preceding attention task. Neuropsychological effects beyond the fMRI-paradigm were assessed by measuring alertness and divided attention. Comparing preceding attention periods of salient vs. neutral pictures, we revealed congruent effects of both drugs vs. placebo within the anterior midcingulate cortex, dorsolateral prefrontal cortex, anterior prefrontal cortex, superior temporal gyrus, anterior insula and the thalamus. Relatively decreased activation in this network was paralleled by slower reaction times in the divided attention task in both verum conditions compared to placebo. Our results suggest similar effects of antidepressant treatments on behavioural and neural attentional functioning by diverging neurochemical pathways. Concurrent alterations of brain regions within a fronto-parietal and cingulo-opercular attention network for top-down control could point to basic neural mechanisms of antidepressant action irrespective of receptor profiles.
Introduction
Antidepressant agents exhibit broad effects on various domains of human experience and behaviour such as mood, anxiety, emotion and reward processing as well as cognitive functions. Although various psychotropic effects e.g. on affect (Knutson et al., 1998; Furlan et al., 2004) or anxiety (Bailey et al., 2007; Boz et al., 2007) have been investigated, data on antidepressant-related effects on cognitive functioning are inconsistent and functional imaging studies on this topic are scarce (Serretti et al., 2010).
We recently investigated the effects of subchronic administration of bupropion and paroxetine in healthy subjects and revealed different effects of the two antidepressants regarding the processing of erotic stimuli in comparison to placebo (Abler et al., 2011). In line with the notion that sexual arousal comprises various components, distinct behavioural measures and brain networks previously related to different domains of affective and cognitive processing were shown to be modulated. As expected from clinical observations (Serretti and Chiesa, 2009), paroxetine, a selective serotonin reuptake-inhibitor (SSRI), was associated with decreased subjective sexual function and decreased neural activations in regions attributed to motivational and emotional processes, whereas bupropion, a selective dopamine and noradrenaline reuptake-inhibitor (SDNRI), led to enhanced neural activations within brain regions related to the processing of reward and salient stimuli and left subjective sexual functioning unchanged. Beside this opposite effect in these neural networks, both drugs led to decreased blood-oxygen level dependent (BOLD) activations within the anterior midcingulate cortex (aMCC) as compared to placebo. MCC activations have been previously associated with a fronto-parietal network (FPN) for top-down control, which mainly initiates and adjusts cognitive control to direct selective attention (Dosenbach et al., 2008). Along with the finding of concurrent modulatory effects of both antidepressants on reaction times in a divided attention (DA) task (Abler et al., 2011), the attenuation of aMCC brain activation was suggested to relate to a congruent modulation of attentional processes under the two antidepressants with otherwise incongruent effects. To further investigate the modulation of cognitive aspects in the context of erotic stimulation by antidepressant agents and particularly effects on preparatory attention related processing, we used an established picture paradigm within the same study sample. The implementation of anticipatory periods was assumed to be suited to assess cognitive attentional processes (Walter et al., 2009).
In general, anticipation (expectancy) can be regarded as preceding attention to an upcoming predicted stimulus (Bermpohl et al., 2006c; Herwig et al., 2007). Using neuroimaging, the cingulate cortex, parieto-occipital sulcus and superior/middle temporal gyrus were suggested to constitute a network specific for expectancy as compared to stimulus perception (Bermpohl et al., 2006c). Furthermore, activation of cingulate brain areas has been linked to sustained attention during expectancy and to a ‘set activity’ for subsequent processing (Russo et al., 2002; Herwig et al., 2007). The suggested parallels between anticipatory and attentional processes are also supported by Corbetta and Shulman (2002). They described a dorsal FPN encompassing the dorsal posterior parietal cortex along the intra-parietal sulcus and the frontal cortex/frontal eye field that controls visuo-spatial, goal-directed attention and primarily responds when subjects attend to a location in anticipation of stimuli. This network is again part of the earlier mentioned fronto-parietal attention network described by Dosenbach et al. (2006). While these studies involved implicit anticipatory attentional processes, Walter et al. (2009) investigated preceding attention in a task that involved explicit anticipation of sexual, emotional and neutral stimuli with an erotic picture task. Enhanced neural activations were present in cortical regions encompassing the dorsal attention network including the dorsolateral prefrontal cortex (dlPFC), the premotor and supplementary motor areas, the anterior and posterior cingulate cortex (ACC, PCC), the anterior insula/frontal operculum and occipito-parietal cortex during expectancy periods, again suggesting an overlap of networks related to attentional and anticipatory processes.
Based on our previous findings using a video task that suggested a unidirectional modulation of attentional processes by paroxetine and bupropion in comparison to placebo, we attempted to further investigate the antidepressant-related modulation of related processes upon the anticipation of stimuli. As in our previous analysis, we expected a unidirectional modulation of neural activation in the aMCC by bupropion and paroxetine. Moreover, we predicted antidepressant-related attenuations in other parts of attention networks e.g. within the dlPFC as a key region within the neural circuits affected in major depression (Mayberg, 2003) and its relation to executive, cognitive and attentional control (MacDonald et al., 2000; Ottowitz et al., 2002; Dosenbach et al., 2006; Herwig et al., 2007).
Method and materials
Subjects
A total of 18 healthy heterosexual, male subjects [mean age 25.4 (s.d. 2.9) yr] were randomized to take placebo, bupropion and paroxetine for 7 d each in a counterbalanced order. Each participant received a full medical evaluation prior to the study including a medical history, a physical examination and a structured clinical interview for DSM-IV Axis I psychiatric disorders. Laboratory blood tests and an electrocardiogram were performed to exclude renal, hepatic or cardiac preconditions. Subjects with any current or past psychiatric or neurological disorders, any serious general medical condition, use of illegal drugs and excessive consumption of caffeine or alcohol (>14 units/wk) were excluded. Further exclusion criteria were clinically relevant baseline sexual dysfunction. Subjects were asked to refrain from alcohol parallel to the study medication and from coffee on the day of the scans. Prior to the study, participants completed a self-report scale regarding depressive symptoms [(CES-D scale; Radloff, 1977) in its German-version Allgemeine Depressionsskala (ADS; Hautzinger and Bailer, 1993)]. The study was approved by the local ethical committee of Ulm University and all volunteers gave written informed consent prior to the study conforming to the Declaration of Helsinki.
Study design and procedures
The study was performed within a randomized double-blind placebo-controlled within-subject cross-over design. Subjects were asked to take paroxetine (20 mg), bupropion (150 mg) and placebo for 7 d each to reach steady state plasma-level conditions. The fMRI-scans took place on day 7 of medication 2 h after intake of the last capsule. Thus, each subject was investigated on three different occasions at intervals of at least 14 d washout-time (for details, see Abler et al., 2011). To estimate drug exposure and adherence, blood samples were taken after each fMRI-session (about 3 h after last drug intake) and analysed after completion of the whole study. The mean plasma bupropion-level of the included subjects was 59.9 ng/ml (s.d. 27.7); the mean plasma paroxetine-level was 28.1 ng/ml (s.d. 17.9).
fMRI task and stimuli
We used an established erotic picture paradigm as described by Walter et al. (2007, 2008b), that has been demonstrated to reliably induce sexual arousal and elicit neural responses in brain areas relevant for sexual and emotional arousal. Moreover, the paradigm was shown to be suitable for the investigation of attentional processes by implementation of anticipatory periods (Walter et al., 2009). Stimuli comprised 20 erotic and 20 non-erotic pictures of positive emotional content taken from the International Affective Picture System (IAPS; Lang et al., 2005). Erotic pictures depicted heterosexual couples in erotic poses; control pictures showed people engaging in emotionally laden, but non-erotic activities. Pictures were matched for standard values of arousal, pleasantness and dominance as provided from the IAPS and for sexual intensity as described before (Walter et al., 2008b). Stimuli were presented for 4 s (picture perception period) followed by a variable interstimulus interval with presentation of a white fixation-cross for 7.5–10.5 s. Half, i.e. 10 stimuli of each type were announced by the presentation of an arrow at a jittered duration of 3–5 s. Downward arrows preceded erotic, upward arrows non-erotic pictures (Fig. 1). Anticipation periods were defined as time between the presentation of an arrow and the beginning of the picture stimuli. The duration of the whole task was 10 min 8 s. After scanning, subjects were asked to rate each of the erotic and non-erotic stimuli for the capacity to induce subjective sexual arousal on a scale from 1 to 9 (1 = not sexually arousing at all; 9 = very sexually arousing).
Erotic-picture paradigm. Subjects were instructed to passively view 20 erotic and 20 non-erotic pictures selected from the International Affective Picture System. Ten pictures of each kind were announced by arrows. Downward arrows (3–5 s) indicated subsequent erotic pictures, whereas non-erotic pictures were announced by upward arrows (3–5 s). Participants were instructed to build up expectancy according to the cue. Picture presentation for 4 s was followed by a variable interstimulus interval with presentation of a fixation cross for 7.5–10.5 s.
fMRI acquisition and procedures
Imaging data were acquired using a 3T head-only MRI system (Siemens Magnetom Allegra, Germany). T2-weighted MR-images were obtained using gradient echo-planar imaging. In-planar matrix size was 64 × 64 with a field of view of 192 mm. The volume consisted of 23 transversal slices (repetition time = 1500 ms; echo time = 35 ms; flip angle 90°) with a slice-thickness of 3 mm and a gap of 0.75 mm (voxel size 3 × 3×3.75 mm) centred on basal structures of the brain including subcortical regions of interest (basal ganglia, midbrain, prefrontal regions). For each session, 552 volumes were acquired. High resolution T1-weighted anatomical images were obtained using three-dimensional magnetization-prepared rapid acquisition with gradient echo sequences.
fMRI analysis
Image pre-processing and statistical analyses were carried out using statistical parametric mapping (SPM5; Wellcome Department, UK) with a random-effects model for group analyses. Data from each session were pre-processed including slice-timing, realignment and normalization into a standard template (Montreal Neurological Institute, Canada). Smoothing was applied with an 8-mm FWHM isotropic Gaussian kernel. Intrinsic autocorrelations were accounted for by AR(1) and low frequency drifts were removed via high-pass filtering.
First-level analyses were performed on each subject estimating the variance of voxels for each trial according to a general linear model. We defined separate regressors for the expectancy (erotic/non-erotic) and for the picture presentation periods. Anticipation periods and picture trials were modelled as timely extended events and convolved with the haemodynamic response function. The six realignment parameters modelling residual motion were added to the design matrix. Individual contrast images for the erotic and non-erotic condition were included in the second-level analysis. To test on significant interaction effects of medication on anticipation of erotic vs. non-erotic stimuli, we computed a two-factorial analysis of variance (ANOVA) with the factors condition (expectancy erotic stimuli, expectancy non-erotic pictures) and treatment (placebo, bupropion, paroxetine). A similar ANOVA was computed for the stimulus presentation period. A mask was computed from the results of an omnibus F test on condition × treatment interactions within the 2 × 3 ANOVA at a statistical threshold of p < 0.005 uncorrected. Post hoc paired t-contrasts on the voxels significant in the 2 × 3 ANOVA were then used to analyse the directionality of the respective treatment effects. Significant effects were inferred when results of the post hoc tests survived a statistical threshold of p < 0.05 (false discovery rate-corrected) and significant clusters comprised at least 10 contiguously significant voxels.
Neuropsychological task and additional questionnaires
To evaluate specific variances in attention beyond fMRI-scanning, we used two subtests of the test for attentional performance (Zimmermann and Fimm, 1993) on each experimental day. The alertness task consists of two simple stimulus detection subtasks, one with (alerted) and one without (self-paced) a warning tone prior to the occurrence of the target stimulus on the screen. The DA task involves reaction to distinct visual and auditory stimuli and requires the ability to attend to simultaneously on-going processes. Both tasks were applied to evaluate general medication effects on attention as a potential moderator of treatment effects. Moreover, subjects completed a modified German version (Reinecke and Hoyer, 2006) of the Massachusetts General Hospital Sexual Function Questionnaire (MGH-SFQ; Labbate and Lare, 2001) to assess sexual functioning over the previous week of medication (Abler et al., 2011). Medication side-effects were assessed by one of the study physicians within a medical interview with open questions and a structured part (UKU side-effects scale; Lingjaerde et al., 1987). To assess potential differences in sedation between each treatment condition, subjects filled the Stanford sleepiness scale (SSS; Hodes et al., 1973). Repeated measures ANOVAs and post hoc paired t tests were computed to analyse reaction time and questionnaire results.
Results
Questionnaires, neuropsychological data and subjective ratings
A mean ADS-scale score of 6.9 (s.d. 5.0) confirmed that none of the participants suffered from clinically relevant depression symptoms. None of the subjects suffered from severe side-effects and subjective ratings of sedation (SSS) did not differ significantly between treatments (one-way ANOVA: F2,34 = 2.06; p = 0.14). Subjective sexual functioning was lower under paroxetine than under placebo but not bupropion (Abler et al., 2011). Further details on specific side-effects (UKU) and results of the MGH-SFQ are summarized in the Supplementary Material.
Ratings regarding subjective sexual arousal for erotic pictures (placebo mean = 5.10 s.d. 1.61; bupropion mean = 5.23 s.d. 1.45; paroxetine mean = 5.01 s.d. 1.66) were significantly (p < 0.05) higher than for non-erotic pictures (placebo mean = 1.41 s.d. 0.50; bupropion mean = 1.36 s.d. 0.51; paroxetine mean = 1.32 s.d. 0.46) in all treatment conditions. However, ratings of erotic-pictures did not differ significantly between verum and placebo (F2,34 = 0.55, p = 0.58).
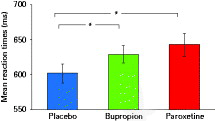
A one-way ANOVA for repeated measures on mean reaction times in the alertness task revealed no significant differences between treatments, neither in the alerted (F2,34 = 0.65, p = 0.53) nor in the self-paced condition (F2,34 = 0.11, p = 0.89). However, in the DA task, we revealed a significant effect of medication (F2,34 = 4.40, p = 0.02) and mean reaction times under placebo were significantly faster than under both antidepressants. Bupropion and paroxetine did not differ significantly (Fig. 2).
Mean reaction times (ms) with s.e.m. observed from the divided attention task. * Indicates significant (p < 0.05) differences in t tests on mean reaction times (placebo vs. bupropion: t17 = 2.46, p = 0.02; placebo vs. paroxetine: t17 = 3.11, p = 0.006; bupropion vs. paroxetine t17 = 0.78, p = 0.459).
fMRI results
By contrasting neural activations during the expectation period of erotic vs. non-erotic emotional pictures under placebo, we confirmed the reliability of the task. As in previous studies with the same task (Bermpohl et al., 2006b; Walter et al., 2007, 2008a, b, 2009), we found elevated BOLD-signals in the ACC, MCC and PCC, the middle frontal gyrus [Brodman area (BA) 9/10/46], insula, medial and superior temporal gyrus (BA21/22), inferior parietal lobe (BA40), fusifom gyrus as well as in subcortical regions such as in the midbrain, the thalamus and in the hippocampus (see Supplementary Table S1). Similarly, results from the picture presentation period under placebo coincided with findings from previous investigations (see Supplementary Table S2).
Treatment effects on anticipation period
A two-factorial ANOVA revealed significant condition × treatment effects within a large set of cortical and subcortical regions during anticipation periods as summarized in Table 1. Directed post hoc t-contrasts on pairwise differences between treatment levels revealed significant BOLD-signal decreases in the pregenual ACC, aMCC, PCC, raphe nuclei, the ventrolateral thalamus, dlPFC (BA9) and anterior prefrontal cortex (aPFC, BA9/10), the anterior insula, the inferior parietal gyrus (BA40) and anterior and posterior lobe of the cerebellum under both antidepressants compared to placebo. Contrasting paroxetine with placebo, decreased activation was furthermore evident in the superior temporal gyrus (BA22), the precentral gyrus (BA6) and the posterior insula. No significant effects in these regions were observed for bupropion vs. placebo. Neither paroxetine nor bupropion led to increased BOLD-signals in comparison to placebo. Both drugs led to attenuated signals in the dlPFC and aPFC compared to placebo, with even less activation under bupropion than under paroxetine.
Significant condition × treatment interaction effects
| Expectancy erotic vs. non-erotic stimuli . | L/R . | Placebo > paroxetine . | Placebo > bupropion . | Bupropion > paroxetine . | Paroxetine >bupropion . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T . | NV . | x/y/z . | T . | NV . | x/y/z . | T . | NV . | x/y/z . | T . | NV . | x/y/z . | ||
| Cingulate cortex | |||||||||||||
| Pregenual ACC, BA32 | L | 3.73 | 48 | −4/40/ − 6 | |||||||||
| Anterior MCC, BA24 | L | 3.43 | 14 | −12/26/24 | 3.53 | 14 | −12/26/24 | ||||||
| R | 4.46 | 51 | 8/24/24 | 3.16 | 51 | 8/26/26 | |||||||
| PCC, BA23 | L | 4.36 | 90 | −10/ − 50/22 | 2.12 | 33 | −6/ − 46/20 | ||||||
| Subcortical structures | |||||||||||||
| Raphe nuclei | 3.41 | 148 | 6/ − 26/ − 26 | 4.21 | 151 | 4/ − 26/ − 26 | |||||||
| Ventrolateral thalamus | L | 3.62 | 97 | −8/ − 10/2 | 3.82 | 97 | −12/ − 12/6 | ||||||
| Cortical activations | |||||||||||||
| Precentral gyrus, BA6 | R | 3.55 | x | 58/2/14 | 3.26 | 26 | 60/2/16 | ||||||
| Gyrus frontalis medius, BA6 | L | 2.82 | 23 | −18/8/46 | 4.54 | 24 | −22/6/44 | ||||||
| Gyrus frontalis superior, BA8/9 | L | 4.18 | 27 | −24/46/38 | 2.80 | 27 | −24/46/38 | ||||||
| dlPFC, BA9 | R | 2.97 | 295 | 28/24/38 | 5.43 | 931 | 32/26/38* | 4.13 | 285 | 34/26/36 | |||
| Middle frontal gyrus, BA9/10 | R | 2.84 | 122 | 40/50/14 | 5.14 | # | 36/48/10* | 3.92 | 360 | 28/52/12 | |||
| Anterior insula | L | 3.57 | 20 | −28/18/10 | |||||||||
| Posterior insula | R | 3.71 | 32 | 38/10/ − 6 | 2.58 | 16 | 38/14/ − 8 | 3.81 | 73 | 38/ − 12/12 | |||
| Lobus parietalis inferior, BA40 | R | 3.37 | 69 | 36/ − 14/12 | |||||||||
| Superior temporal gyrus, BA22 | R | 4.21 | 113 | 46/ − 28/26 | 3.72 | 86 | 48/ − 24/26 | 3.29 | 22 | 44/ − 36/36 | |||
| L | 4.43 | 94 | −50/ − 36/16 | 2.83 | 60 | −44/ − 38/26 | |||||||
| R | 5.16 | 354 | 56/ − 16/0* | 4.43 | 66 | 54/ − 16/0 | |||||||
| L | 3.67 | 14 | −50/ − 16/ − 2 | ||||||||||
| Cerebellum | L | 4.03 | 39 | −24/ − 40/ − 26 | 3.95 | 39 | −22/ − 40/ − 24 | ||||||
| R | 4.53 | 148 | 14/ − 36/ − 28 | 3.38 | 11 | 14/ − 46/ − 16 | 3.22 | 11 | 16/ − 46/ − 16 | ||||
| Expectancy erotic vs. non-erotic stimuli . | L/R . | Placebo > paroxetine . | Placebo > bupropion . | Bupropion > paroxetine . | Paroxetine >bupropion . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T . | NV . | x/y/z . | T . | NV . | x/y/z . | T . | NV . | x/y/z . | T . | NV . | x/y/z . | ||
| Cingulate cortex | |||||||||||||
| Pregenual ACC, BA32 | L | 3.73 | 48 | −4/40/ − 6 | |||||||||
| Anterior MCC, BA24 | L | 3.43 | 14 | −12/26/24 | 3.53 | 14 | −12/26/24 | ||||||
| R | 4.46 | 51 | 8/24/24 | 3.16 | 51 | 8/26/26 | |||||||
| PCC, BA23 | L | 4.36 | 90 | −10/ − 50/22 | 2.12 | 33 | −6/ − 46/20 | ||||||
| Subcortical structures | |||||||||||||
| Raphe nuclei | 3.41 | 148 | 6/ − 26/ − 26 | 4.21 | 151 | 4/ − 26/ − 26 | |||||||
| Ventrolateral thalamus | L | 3.62 | 97 | −8/ − 10/2 | 3.82 | 97 | −12/ − 12/6 | ||||||
| Cortical activations | |||||||||||||
| Precentral gyrus, BA6 | R | 3.55 | x | 58/2/14 | 3.26 | 26 | 60/2/16 | ||||||
| Gyrus frontalis medius, BA6 | L | 2.82 | 23 | −18/8/46 | 4.54 | 24 | −22/6/44 | ||||||
| Gyrus frontalis superior, BA8/9 | L | 4.18 | 27 | −24/46/38 | 2.80 | 27 | −24/46/38 | ||||||
| dlPFC, BA9 | R | 2.97 | 295 | 28/24/38 | 5.43 | 931 | 32/26/38* | 4.13 | 285 | 34/26/36 | |||
| Middle frontal gyrus, BA9/10 | R | 2.84 | 122 | 40/50/14 | 5.14 | # | 36/48/10* | 3.92 | 360 | 28/52/12 | |||
| Anterior insula | L | 3.57 | 20 | −28/18/10 | |||||||||
| Posterior insula | R | 3.71 | 32 | 38/10/ − 6 | 2.58 | 16 | 38/14/ − 8 | 3.81 | 73 | 38/ − 12/12 | |||
| Lobus parietalis inferior, BA40 | R | 3.37 | 69 | 36/ − 14/12 | |||||||||
| Superior temporal gyrus, BA22 | R | 4.21 | 113 | 46/ − 28/26 | 3.72 | 86 | 48/ − 24/26 | 3.29 | 22 | 44/ − 36/36 | |||
| L | 4.43 | 94 | −50/ − 36/16 | 2.83 | 60 | −44/ − 38/26 | |||||||
| R | 5.16 | 354 | 56/ − 16/0* | 4.43 | 66 | 54/ − 16/0 | |||||||
| L | 3.67 | 14 | −50/ − 16/ − 2 | ||||||||||
| Cerebellum | L | 4.03 | 39 | −24/ − 40/ − 26 | 3.95 | 39 | −22/ − 40/ − 24 | ||||||
| R | 4.53 | 148 | 14/ − 36/ − 28 | 3.38 | 11 | 14/ − 46/ − 16 | 3.22 | 11 | 16/ − 46/ − 16 | ||||
L, Left; R, right, T, T value; NV, number of contiguously significant voxels (voxel size after normalization: 2 × 2×2 mm3); x/y/z are peak coordinates in Montreal Neurological Institute space; ACC, anterior cingulate cortex; MCC, midcingulate cortex; dlPFC, dorsolateral prefrontal cortex; PCC, posterior cingulate cortex; BA, Brodman area.
Brain areas with significant interaction effects in the 2 × 3 ANOVA even at the more conservative threshold of p < 0.05, FDR-corrected. No significant interactions were observed for paroxetine > placebo or bupropion > placebo.
Activation is part of cluster above; x, part of a cluster consisting of 354 voxel from superior temporal gyrus extending to the precentral gyrus.
Results are shown of post hoc testing of pairwise differences between treatments at p < 0.05, false discovery rate (FDR)-corrected. The post hoc testing was locally constrained to voxels surviving a threshold of p < 0.005 in the 2 × 3 analysis of variance (ANOVA) with factors condition (expected erotic, expected non-erotic) and treatment.
Significant condition × treatment interaction effects
| Expectancy erotic vs. non-erotic stimuli . | L/R . | Placebo > paroxetine . | Placebo > bupropion . | Bupropion > paroxetine . | Paroxetine >bupropion . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T . | NV . | x/y/z . | T . | NV . | x/y/z . | T . | NV . | x/y/z . | T . | NV . | x/y/z . | ||
| Cingulate cortex | |||||||||||||
| Pregenual ACC, BA32 | L | 3.73 | 48 | −4/40/ − 6 | |||||||||
| Anterior MCC, BA24 | L | 3.43 | 14 | −12/26/24 | 3.53 | 14 | −12/26/24 | ||||||
| R | 4.46 | 51 | 8/24/24 | 3.16 | 51 | 8/26/26 | |||||||
| PCC, BA23 | L | 4.36 | 90 | −10/ − 50/22 | 2.12 | 33 | −6/ − 46/20 | ||||||
| Subcortical structures | |||||||||||||
| Raphe nuclei | 3.41 | 148 | 6/ − 26/ − 26 | 4.21 | 151 | 4/ − 26/ − 26 | |||||||
| Ventrolateral thalamus | L | 3.62 | 97 | −8/ − 10/2 | 3.82 | 97 | −12/ − 12/6 | ||||||
| Cortical activations | |||||||||||||
| Precentral gyrus, BA6 | R | 3.55 | x | 58/2/14 | 3.26 | 26 | 60/2/16 | ||||||
| Gyrus frontalis medius, BA6 | L | 2.82 | 23 | −18/8/46 | 4.54 | 24 | −22/6/44 | ||||||
| Gyrus frontalis superior, BA8/9 | L | 4.18 | 27 | −24/46/38 | 2.80 | 27 | −24/46/38 | ||||||
| dlPFC, BA9 | R | 2.97 | 295 | 28/24/38 | 5.43 | 931 | 32/26/38* | 4.13 | 285 | 34/26/36 | |||
| Middle frontal gyrus, BA9/10 | R | 2.84 | 122 | 40/50/14 | 5.14 | # | 36/48/10* | 3.92 | 360 | 28/52/12 | |||
| Anterior insula | L | 3.57 | 20 | −28/18/10 | |||||||||
| Posterior insula | R | 3.71 | 32 | 38/10/ − 6 | 2.58 | 16 | 38/14/ − 8 | 3.81 | 73 | 38/ − 12/12 | |||
| Lobus parietalis inferior, BA40 | R | 3.37 | 69 | 36/ − 14/12 | |||||||||
| Superior temporal gyrus, BA22 | R | 4.21 | 113 | 46/ − 28/26 | 3.72 | 86 | 48/ − 24/26 | 3.29 | 22 | 44/ − 36/36 | |||
| L | 4.43 | 94 | −50/ − 36/16 | 2.83 | 60 | −44/ − 38/26 | |||||||
| R | 5.16 | 354 | 56/ − 16/0* | 4.43 | 66 | 54/ − 16/0 | |||||||
| L | 3.67 | 14 | −50/ − 16/ − 2 | ||||||||||
| Cerebellum | L | 4.03 | 39 | −24/ − 40/ − 26 | 3.95 | 39 | −22/ − 40/ − 24 | ||||||
| R | 4.53 | 148 | 14/ − 36/ − 28 | 3.38 | 11 | 14/ − 46/ − 16 | 3.22 | 11 | 16/ − 46/ − 16 | ||||
| Expectancy erotic vs. non-erotic stimuli . | L/R . | Placebo > paroxetine . | Placebo > bupropion . | Bupropion > paroxetine . | Paroxetine >bupropion . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T . | NV . | x/y/z . | T . | NV . | x/y/z . | T . | NV . | x/y/z . | T . | NV . | x/y/z . | ||
| Cingulate cortex | |||||||||||||
| Pregenual ACC, BA32 | L | 3.73 | 48 | −4/40/ − 6 | |||||||||
| Anterior MCC, BA24 | L | 3.43 | 14 | −12/26/24 | 3.53 | 14 | −12/26/24 | ||||||
| R | 4.46 | 51 | 8/24/24 | 3.16 | 51 | 8/26/26 | |||||||
| PCC, BA23 | L | 4.36 | 90 | −10/ − 50/22 | 2.12 | 33 | −6/ − 46/20 | ||||||
| Subcortical structures | |||||||||||||
| Raphe nuclei | 3.41 | 148 | 6/ − 26/ − 26 | 4.21 | 151 | 4/ − 26/ − 26 | |||||||
| Ventrolateral thalamus | L | 3.62 | 97 | −8/ − 10/2 | 3.82 | 97 | −12/ − 12/6 | ||||||
| Cortical activations | |||||||||||||
| Precentral gyrus, BA6 | R | 3.55 | x | 58/2/14 | 3.26 | 26 | 60/2/16 | ||||||
| Gyrus frontalis medius, BA6 | L | 2.82 | 23 | −18/8/46 | 4.54 | 24 | −22/6/44 | ||||||
| Gyrus frontalis superior, BA8/9 | L | 4.18 | 27 | −24/46/38 | 2.80 | 27 | −24/46/38 | ||||||
| dlPFC, BA9 | R | 2.97 | 295 | 28/24/38 | 5.43 | 931 | 32/26/38* | 4.13 | 285 | 34/26/36 | |||
| Middle frontal gyrus, BA9/10 | R | 2.84 | 122 | 40/50/14 | 5.14 | # | 36/48/10* | 3.92 | 360 | 28/52/12 | |||
| Anterior insula | L | 3.57 | 20 | −28/18/10 | |||||||||
| Posterior insula | R | 3.71 | 32 | 38/10/ − 6 | 2.58 | 16 | 38/14/ − 8 | 3.81 | 73 | 38/ − 12/12 | |||
| Lobus parietalis inferior, BA40 | R | 3.37 | 69 | 36/ − 14/12 | |||||||||
| Superior temporal gyrus, BA22 | R | 4.21 | 113 | 46/ − 28/26 | 3.72 | 86 | 48/ − 24/26 | 3.29 | 22 | 44/ − 36/36 | |||
| L | 4.43 | 94 | −50/ − 36/16 | 2.83 | 60 | −44/ − 38/26 | |||||||
| R | 5.16 | 354 | 56/ − 16/0* | 4.43 | 66 | 54/ − 16/0 | |||||||
| L | 3.67 | 14 | −50/ − 16/ − 2 | ||||||||||
| Cerebellum | L | 4.03 | 39 | −24/ − 40/ − 26 | 3.95 | 39 | −22/ − 40/ − 24 | ||||||
| R | 4.53 | 148 | 14/ − 36/ − 28 | 3.38 | 11 | 14/ − 46/ − 16 | 3.22 | 11 | 16/ − 46/ − 16 | ||||
L, Left; R, right, T, T value; NV, number of contiguously significant voxels (voxel size after normalization: 2 × 2×2 mm3); x/y/z are peak coordinates in Montreal Neurological Institute space; ACC, anterior cingulate cortex; MCC, midcingulate cortex; dlPFC, dorsolateral prefrontal cortex; PCC, posterior cingulate cortex; BA, Brodman area.
Brain areas with significant interaction effects in the 2 × 3 ANOVA even at the more conservative threshold of p < 0.05, FDR-corrected. No significant interactions were observed for paroxetine > placebo or bupropion > placebo.
Activation is part of cluster above; x, part of a cluster consisting of 354 voxel from superior temporal gyrus extending to the precentral gyrus.
Results are shown of post hoc testing of pairwise differences between treatments at p < 0.05, false discovery rate (FDR)-corrected. The post hoc testing was locally constrained to voxels surviving a threshold of p < 0.005 in the 2 × 3 analysis of variance (ANOVA) with factors condition (expected erotic, expected non-erotic) and treatment.
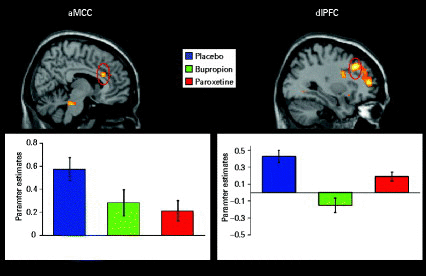
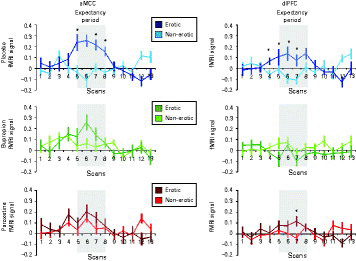
Figure 3 shows the signal from two prototypical regions with significant interaction effects during the anticipation period. The aMCC was chosen as the only brain region that equally shows decreased activation under both antidepressants upon expectancy and before direct stimulation with erotic video clips (Abler et al., 2011). The dlPFC seemed interesting as a representative brain region with attenuated signals under both drugs but still higher neural activations under paroxetine compared to bupropion and as a key region related to expectancy and depression (Herwig et al., 2007; Walter et al., 2009). To further illustrate the drug effects within these regions, signal time-courses were extracted (Fig. 4).
Significant differential functional magnetic resonance imaging activations (analysis of variance for demonstration purposes: p < 0.005, uncorrected) during the anticipation of erotic vs. non-erotic pictures in the anterior midcingulate cortex (aMCC), the right-sided dorsolateral prefrontal cortex (dlPFC, BA9) and medio-frontal gyrus (BA9/10). Plots show parameter estimates of modelled effects averaged over the voxels of the cluster (aMCC, dlPFC) at a statistical threshold of p < 0.05, false discovery rate-corrected.
Extracted signal time-courses (vertical bars, s.e.m.) within the anterior midcingulate cortex (aMCC) and right-sided dorsolateral prefrontal cortex (dlPFC). The time-courses were averaged event-related to depict the functional magnetic resonance (MR) imaging-signal related to the anticipation of erotic and non-erotic pictures in the placebo, bupropion and paroxetine sessions. Expectancy periods began at the scan time-point 1 in with a variable length of 3–5 s (equally 2–3.33 scans, TR = 1.5 s). Grey shades indicate the period when the MR-signal related to an exemplary expectation period with a duration of 3 s is expected to occur, assuming a delay of the haemodynamic response of 6 s (4 TRs). Time-courses right of the grey shades contribute to the stimulus presentation period. The mean-corrected first eigenvariate values were extracted from the aMCC and dlPFC for each subject for verum and placebo. The aMCC cluster was extracted at a statistical threshold of p < 0.005, uncorrected for demonstrational purposes. Since the dlPFC-cluster showed a broad extension into the anterior prefrontal region at this statistical threshold, dlPFC-signal time-courses were extracted at a more conservative threshold of p < 0.05, false discovery rate-corrected to allow reasonable comparisons. * Indicates significant differences of differential blood-oxygen level dependent signals (erotic vs. non-erotic) in paired t tests (p < 0.05) for demonstrational purposes.
Treatment effects on stimulus perception
The two-factorial ANOVA with the main factors condition (perception of erotic/non-erotic pictures) and treatment (placebo, paroxetine, bupropion) revealed no significant treatment effects on neural activations during stimulus presentation at the predefined statistical thresholds. Only at a more lenient statistical threshold (p < 0.005, uncorrected), we revealed treatment effects within the dorsomedial prefrontal cortex and right inferior frontal cortex under bupropion and paroxetine in comparison to placebo with no differences between the drugs. Detailed analyses (ANOVA) of treatment effects contrasting all erotic minus all non-erotic pictures in the expected mode as well as unexpected erotic minus unexpected non-erotic pictures revealed also no significant results for the stimulus presentation period.
Correlation analyses
Since we revealed decreased brain activations during preceding anticipation under both drugs within the aMCC and dlPFC in comparison to placebo contrasting erotic vs. non-erotic expectancy, we hypothesized that these concurrent effects relate to concurrent drug-effects in neuropsychological testing. Therefore, we post hoc correlated β-values from anticipation periods in the earlier mentioned prototypical regions. This revealed a significant negative correlation between DA reaction times and brain activation within the dlPFC under paroxetine (r = − 0.493, p = 0.019, Fisher Z = 0.540). Faster subjects with relatively short reaction times showed greater brain activation. However, those subjects with the greatest increase of reaction times under paroxetine vs. placebo in the DA task showed a trend to the greatest decrease of brain activation related to paroxetine in the aMCC (r = − 0.380, p = 0.060, Fisher Z = 0.400).
Discussion
Our investigations within a double-blind, placebo-controlled cross-over design revealed a unidirectional modulation of neural networks contributed to preceding attention on salient stimuli by different types of antidepressants that showed diverging effects on networks related to affective vs. cognitive processes in a previous study (Abler et al., 2011). Likewise, mean reaction times increased in a DA task under both drugs bupropion and paroxetine, compared to placebo. Whereas BOLD-signals remained unchanged during stimulus perception, we observed a significant unidirectional attenuation of fMRI-activations under both drugs within the ACC (pregenual ACC, aMCC), adding to a network which further comprised the dlPFC and aPFC, the anterior insula, the inferior parietal gyrus and the ventrolateral thalamus. Decreased dlPFC-activation was significantly correlated with decelerated reaction times in DA under paroxetine, whereas a trend to correlation with differential reaction times was observed within the aMCC.
Main effects on stimulus perception and during anticipation of salient stimuli
Confirming the reliability of the task, the investigation of neural effects of placebo on anticipatory cognitive processes and perception of a sexual stimulus revealed highly similar networks to previous studies without any drug application (Supplementary Table S1/S2; Simmons et al., 2004; Bermpohl et al., 2006c; Safron et al., 2007; Walter et al., 2008a, 2009). In line with investigations on dissociable networks for anticipation and stimulus perception within a comparable picture paradigm, elevated neural signals within the cingulate cortex (BA24/BA32), the parieto-occipital sulcus (BA7/BA19/BA31) and the superior/middle temporal gyrus (BA21/BA22) were specifically observed during expectancy but not for stimulus perception (Bermpohl et al., 2006c).
Modulation of attention networks during anticipation – an effect independent of antidepressant receptor profiles
As hypothesized from previous findings, both antidepressants, the SSRI and the SDNRI, displayed a concurrent modulation of networks related to attentional processes upon anticipation of salient stimuli. From a neuropsychological perspective, anticipation and attention are two cognitive functions immediately linked together. During expectancy periods, neural prestimulus activity is thought to facilitate a ‘set-activity’ to direct the subject's attention to a subsequent stimulus and to sustain attention to allow for an adequate adaption of behaviour (Russo et al., 2002; Herwig et al., 2007). Related to this, Corbetta and Shulman (2002) described a dorsal attention network that controls goal-directed attention when subjects deliberately direct their attention in anticipation of a stimulus. Investigations on preceding attention with the same task as used in our study (Walter et al., 2009) support the close conjunction between anticipation and attentional processes by describing enhanced neural activations within the aforementioned dorsal attention network during anticipation. The regions involved largely overlap with those modulated by both drugs used in our study.
Recent meta-analyses refined the view on neural attention networks for top-down control (Dosenbach et al., 2006, 2007, 2008). Whereas a FPN consisting of the dlPFC, the MCC, the inferior parietal lobule, the dorsal frontal cortex, the intra-parietal sulcus and the precuneus mainly initiates and adjusts control, the cingulo-opercular network (CON) encompassing the aPFC, the anterior insula/frontal operculum, the dorsal ACC, the medial superior frontal cortex and the thalamus is crucial for the maintenance of attention. In comparison to placebo, we observed treatment effects within regions that are part of the FPN (in our study: aMCC; dlPFC; inferior parietal lobe; superior temporal gyrus) but both antidepressant agents also led to unidirectional attenuations of brain activation in regions within the CON (in our study: aPFC BA9/10; anterior insula; thalamus; medial superior frontal cortex BA6/8/9). Treatment effects during the anticipation phase may therefore relate to a modulation of sustained attention, in terms of diminished neural activities in the CON as well as to task initiation, in terms of the reduced neural activities within the FPN which overlaps with the former proposed dorsal attention network (Corbetta and Shulman, 2002; Dosenbach et al., 2008). The interpretation of evident treatment effects on attentional control processes is not only supported from a cerebral network perspective but also by the behavioural finding of concurrent effects of both antidepressants on DA and correlations between brain activation and behaviour. The DA task puts subjects in a dual-task situation that requires the maintenance but also the shift of attentional resources and different task sets. Since reaction times in the simple alertness task were unaffected, one might speculate that both drugs led to a specific modulation of attentional processes like task-shifts. As this occurred independently of different receptor profiles, this may suggest a core effect of antidepressant treatments. Increased brain activation particularly in medial frontal regions has been described to relate to non-beneficially increased self-referential processing such as rumination in depression (Drevets et al., 2008; Gotlib and Hamilton, 2008). The activation decreases under the drugs revealed by our study could therefore represent a part of the drugs actual mechanism of action, similar to those found in the context of emotion processing (Godlewska et al., 2012).
A crucial role in the network may be mediated by the aMCC of which activation was found to be modulated by both paroxetine and bupropion in a previous study (Abler et al., 2011) and now replicated upon anticipation of picture stimuli. The aMCC has been discussed as central for wilful, deliberate control of behaviour (Paus, 2001) and shows functional associations with cognitive, salience and affective networks (Yu et al., 2011) and also with reward processing (Vogt et al., 2003). Previous studies have already shown diminished neural activations under 7-d SSRI-treatment in the context of a reward network (McCabe et al., 2010) and emotional stimulus processing (Harmer et al., 2006). Moreover, as a central node of the FPN (Corbetta and Shulman, 2002), MCC activations has been associated with starting cue-related activity and directing selective attention (Dosenbach et al., 2007), both required during anticipatory periods. The lack of significant treatment effects during stimulus perception and alterations of DA correlating to brain activation are well in line with our hypothesis that the observed concurrent drug effects contribute more to cognitive than to affective domains.
The dlPFC is another candidate for a core node for antidepressant action with its known role in general executive and cognitive control mechanisms (MacDonald et al., 2000; Ottowitz et al., 2002; Herwig et al., 2007) including attentional top-down control (Dosenbach et al., 2006) as well as during expectancy (Walter et al., 2009). Here, modulation was even stronger under bupropion than under paroxetine. The functioning of the dlPFC has been shown to be sensitive to levels of catecholamines. The relationship has been conceptualized as an inverted U-shaped curve (Arnsten, 2011) whereby either too low or too high levels of either dopamine (Dreher et al., 2002) or noradrenaline (Graf et al., 2011) led to a worsening of PFC functioning. One might argue that an increase in noradrenergic and dopaminergic neurotransmission beyond the optimum in healthy subjects may have led to decreased neural signalling within this region and to slower reaction times as measured by the DA task. Further investigations using varying doses of bupropion could help to verify this hypothesis. Taking into account that not only the SDNRI bupropion but also paroxetine increases the dopaminergic neurotransmission in prefrontal areas via indirect pathways (overview in Owen and Whitton, 2006; Lavergne and Jay, 2010) makes similarly altered activations under paroxetine correlating with behavioural changes in DA plausible. Besides unidirectionally altered prefrontal functioning, the reported initial reduction of noradrenergic transmission in brain stem nuclei not only under bupropion (El Mansari, 2008) but also under SSRIs (Pineyro and Blier, 1999; Szabo et al., 1999, 2000; Grant and Weiss, 2001) could help to explain the concurrently altered neural signalling within attention-related brain regions, e.g. in the dlPFC and impaired DA. Executive functions like attention depend on prefrontal noradrenaline with an inverted U-shape relationship (Aston-Jones and Cohen, 2005; Cain et al., 2011).
Together with the primary contribution of the noradrenergic system on attentional control (for review see Noudoost and Moore, 2011), cognitive functions such as cognitive flexibility and attention have been also linked to serotonergic neurotransmission (Schmitt et al., 2006). Thus, the similar effects on the behavioural level and also regarding brain activation could be a single product of two different pathways. However, the influence of SSRIs on different aspects of attention remains rather inconsistent. In line with our results, altered sustained attention has previously been observed in healthy subjects under SSRIs with the Mackworth–Clock paradigm (Ramaekers et al., 1995; Schmitt et al., 2002; Riedel et al., 2005; Wingen et al., 2008). Wingen et al. (2008) investigated the influence of increased serotonin levels on brain areas involved in sustained attention and observed reduced neural activations in the thalamus, supplementary motor area and middle, inferior and superior frontal gyrus as well as in the superior temporal gyrus after administration of escitalopram. This activation pattern mainly overlaps again with the CON described by Dosenbach et al. (2008) and is in good accordance with our results, thus suggesting an alternative explanation for the modulation of attention networks under SSRI administration in healthy subjects.
Clinical implications
Although pharmacological alterations in brain activity in healthy subjects may differ from those in patients due to divergent baseline levels, some basic principles explaining clinical effects might also be derived from investigations in healthy subjects. The right-sided dlPFC of which activation was decreased by both drugs in our study is one of the brain regions that has been frequently suggested to be affected in major depression. Whereas most evidence points toward decreased activity within prefrontal areas in major depression, data are still inconsistent with reports of normal frontal as well as hyperfrontal activity (Mayberg et al., 2000; Fitzgerald et al., 2008). With regard to laterality, recent neuroimaging studies suggested imbalances between the left and right dlPFC in major depression and positron emission tomography-studies revealed reduced cerebral blood flow and metabolism in the left dlPFC and hypermetabolism in the right-sided dlPFC (Mayberg, 2003; Phillips et al., 2003) that entails relevance in therapeutic effects of repetitive transcranial magnetic stimulation (Burt et al., 2002; Gershon et al., 2003; Bermpohl et al., 2006a). This has led to the imbalance hypothesis of major depression which postulates prefrontal hypoactivity in the left dlPFC and hyperactivity in the right dlPFC (Sackeim et al., 1982; Davidson and Irwin, 1999; Maeda et al., 2000). A recent fMRI-study investigated neural activities in the left and right dlPFC related to unexpected and expected judgement of emotions in healthy subjects in comparison to patients with major depressive disorder (Grimm et al., 2008). It was observed that left dlPFC-hypoactivity is associated with negative emotional judgment rather than with perception whereas right dlPFC-hyperactivity is related to anticipation and attention modulation. This is also supported by other investigations (Ueda et al., 2003; Nitschke and Mackiewicz, 2005) and in good accordance with our findings of antidepressant-related decreased BOLD-signals only within the right-sided dlPFC. This might be the mechanism by which both antidepressants reduce prefrontal hyperactivity in major depression that was previously related to increased attention toward negative emotional judgments (Grimm et al., 2008). Concurrent attenuation of the aMCC might occur due to the positively functional (Yu et al., 2011) and anatomical, reciprocal connections with the dlPFC (Barbas and Pandya, 1989). Whether the observed drug-related decreases in brain activation represent an improvement or decrease of cerebral functioning, however, remains a matter of debate (Logothetis, 2008). As discussed for clinical depression, where increased and decreased activations are observed (Drevets et al., 2008; Gotlib and Hamilton, 2008 for reviews) it may depend on the specific context whether a decrease of brain activation is beneficial or not.
Limitations
As we investigated healthy subjects upon a subchronic duration of intake, transferability of our results to patient populations with common treatment periods of several weeks to the onset of antidepressant action is limited. Regarding the design, the varying durations of the expectation periods may prevent habituation effects on the one side, but also might induce different expectation tension levels as a potential confound to the study. Furthermore, our design does not allow for controlling for expectancy-driven placebo effects. However, neural activation patterns observed in the placebo condition largely overlap with activations found without pharmacological intervention (Bermpohl et al., 2006b; Walter et al., 2007, 2008a, b, 2009). Thus, mere placebo effects are less likely to interfere with the interpretability of our results. Another confound may be the induction of a basic psychological state by the drugs causing the networks involved to be utilized differently overall. However, such a basic alteration should equally affect stimulus expectation and presentation periods while we observed drug effects only upon expectation.
Conclusion
We demonstrate that subchronic administration of paroxetine and bupropion leads to alterations of neural network activity within brain regions that have been linked to attentional top-down control, i.e. task initiation and adjustment (FPN) as well as maintenance of attention (CON). These neural network alterations were accompanied by longer reaction times upon DA on a behavioural level, a task that requires both the reorientation and maintenance of an attentional set. Besides their similarly antidepressant action on mood and drive, both drugs are well known for their different side-effects profiles. The concurrent modulation of attention-related neural activity as measured by a preceding attention task irrespective of neurochemical receptor profiles by both drugs may be interpreted in a way that modulation of these networks may represent a core correlate of antidepressant action.
Supplementary material
Supplementary material accompanies this paper on the Journal's website.
Supplementary information supplied by authors.
Supplementary information supplied by authors.
Acknowledgements
We thank Professor Dr C. Hiemke and his staff at the University of Mainz (Germany), Department of Psychiatry and Psychotherapy, for measurements of drug blood serum levels.
Statement of Interest
C. D. M. was supported by DFG-SFB 779 grant.
References
- norepinephrine
- dopamine
- bupropion
- serotonin
- antidepressive agents
- consciousness related finding
- paroxetine
- prefrontal cortex
- reaction time
- thalamus
- behavior
- brain
- reward
- selective serotonin re-uptake inhibitors
- mood
- sexual excitation
- functional magnetic resonance imaging
- adverse effects
- divided attention
- anterior insula