-
PDF
- Split View
-
Views
-
Cite
Cite
Sarah Pol Bodetto, Pascal Romieu, Maxime Sartori, Carolina Tesone-Coelho, Monique Majchrzak, Alexandra Barbelivien, Jean Zwiller, Patrick Anglard, Differential regulation of MeCP2 and PP1 in passive or voluntary administration of cocaine or food, International Journal of Neuropsychopharmacology, Volume 17, Issue 12, December 2014, Pages 2031–2044, https://doi.org/10.1017/S1461145714000972
Close - Share Icon Share
Abstract
Cocaine exposure induces changes in the expression of numerous genes, in part through epigenetic modifications. We have initially shown that cocaine increases the expression of the chromatin remodeling protein methyl-CpG binding protein 2 (MeCP2) and characterized the protein phosphatase-1Cβ (PP1Cβ) gene, as repressed by passive i.p. cocaine injections through a Mecp2-mediated mechanism involving de novo DNA methylation. Both proteins being involved in learning and memory processes, we investigated whether voluntary cocaine administration would similarly affect their expression using an operant self-administration paradigm. Passive and voluntary i.v. cocaine intake was found to induce Mecp2 and to repress PP1Cβ in the prefrontal cortex and the caudate putamen. This observation is consistent with the role of Mecp2 acting as a transcriptional repressor of PP1Cβ and shows that passive intake was sufficient to alter their expression. Surprisingly, striking differences were observed under the same conditions in food-restricted rats tested for food pellet delivery. In the prefrontal cortex and throughout the striatum, both proteins were induced by food operant conditioning, but remained unaffected by passive food delivery. Although cocaine and food activate a common reward circuit, changes observed in the expression of other genes such as reelin and GAD67 provide new insights into molecular mechanisms differentiating neuroadaptations triggered by each reinforcer. The identification of hitherto unknown genes differentially regulated by drugs of abuse and a natural reinforcer should improve our understanding of how two rewarding stimuli differ in their ability to drive behavior.
Introduction
Cocaine exposure is known to induce changes in gene expression in brain structures related to the reward system (Maze and Nestler, 2011). Persistent transcriptional modifications are likely to contribute to neural and behavioral adaptations triggered by the drug. We and others have shown that epigenetic regulators of gene expression are involved in cocaine-altered gene expression as well as in cocaine-related behavior (Cassel et al., 2006; Romieu et al., 2008; Host et al., 2011; Robison and Nestler, 2011). Among epigenetic factors involved, the methyl-CpG binding protein 2 (MeCP2) and histone deacetylase 2 (HDAC2) were identified as two interacting proteins implicated in transcriptional repression through chromatin compaction (Jones et al., 1998; Nan et al., 1998). We have also demonstrated that cocaine triggers changes in DNA methylation by identifying the Cdkl5 gene as the first gene methylated in adult brain in response to cocaine leading to its repression by Mecp2 (Carouge et al., 2010).
The critical involvement of Mecp2 and DNA methylation in the regulation of behavioral responses to psychostimulants was further illustrated by studies showing that: (i) decreased cocaine intake was observed following Mecp2 knockdown in the caudate putamen (CPu) (Im et al., 2010); (ii) amphetamine-conditioned place preference (CPP) and enhanced locomotion were not observed in Mecp2 mutant mice (Deng et al., 2010); (iii) overexpression of the DNA methyltransferase 3A (DNMT3A) in the nucleus accumbens (NAc) reduced cocaine CPP and enhanced depression-like behavior, and conversely its knockdown enhanced cocaine CPP and had anti-depressive effects (LaPlant et al., 2010). We have recently characterized the β catalytic subunit of protein phosphatase-type 1 (PP1Cβ), as an additional gene repressed by repeated passive cocaine administration involving DNA hypermethylation and increased-Mecp2 binding at its promoter region in vivo (Pol Bodetto et al., 2013). PP1 is involved in synaptic and structural plasticity, mediates various signaling pathways (Ceulemans and Bollen, 2004; Mansuy and Shenolikar, 2006) and numerous studies have demonstrated that PP1 and its endogenous inhibitor DARPP-32 (dopamine- and cyclic amphetamine-regulated phosphoprotein) are key actors in cocaine effects (Svenningsson et al., 2004). However, although decrease in PP1 activity is required for cocaine behavioral effects (Hiroi et al., 1999; Zachariou et al., 2006), the role attributed to PP1 mainly results from the use of PP1 endogenous inhibitors such as DARPP-32 or Inhibitor-1 that do not discriminate between PP1C isoforms.
PP1 being implicated in cocaine effects and in learning and memory processes as a negative regulator of memory (Genoux et al., 2002; Miller and Sweatt, 2007; Graff et al., 2010; Koshibu et al., 2011), we investigated in the present study the regulation of PP1Cβ expression by voluntary drug intake using a cocaine self-administration paradigm, as compared to passive cocaine administration. As drugs of abuse and natural rewards activate common brain regions and reward circuitry, we also investigated whether food reinforcement could differentially regulate the expression of PP1Cβ Mecp2 and HDAC2 compared to cocaine. The aim of the study was to find out whether these genes playing important roles in common cognitive functions could respond and therefore contribute differently to specific neuroadaptations in order to better understand the molecular mechanisms triggered by each reward.
Materials and methods
Animals
Male Wistar rats (Janvier, France), weighing 160–180 g, were housed individually in standard home cages, in a temperature- and humidity-controlled room, under an inverted 12 h/12 h light/dark cycle (lights on at 19:00 hours). Animals were acclimated to laboratory conditions and handled one week before experimental procedures. Each behavioral experiment started 3 wk after their arrival in the laboratory and was conducted during the dark period. Animals used for the cocaine operant self-administration experiment (n = 28) had ad libitum access to food (12 mm pellets, 3.95 cal/mg, 4RF21 GLP, Mucedola srl, Italy) and water, whereas animals used for the food pellets operant self-administration experiment (n = 31) were food restricted. Their body weight was progressively reduced to 85% and was maintained at this level throughout the experiment. All procedures involving animal care were conducted in compliance with national laws and policies (Council directive 87 848, 1987, Service Vétérinaire de la Santé et de la Protection animale, permission 67–165 to JZ) and with international guidelines (NIH publication 5586-23, 1985).
Surgery, apparatus and cocaine operant self-administration
For technical details, see Supplement 1 (available online).
Apparatus and food pellets operant self-administration procedure
Dark five-choice operant chambers (25.2 × 28 × 24 cm, Bioseb, BP 89 92 370 Chaville, France) placed in sound-attenuated and ventilated enclosures were used to test food pellets operant self-delivery. The curved rear wall comprised nine contiguous 2.3 cm square hole, located 2.2 cm above the grid floor. Each hole was equipped with an infrared photocell beam to detect nose pokes (NPs). The two farthest holes were used as the active and the inactive holes, the other holes being obstructed by a metal cover. NPs into both holes were recorded. NPa and NPi were counterbalanced between right and left position in each experimental group and in the various groups of rats. The active hole was associated with the delivery of a food pellet (Precision-Pellets 45 mg, 3.62 cal/mg, 259901-PEL, Bioserv purified formula, TSE systems, Germany) into a magazine located at the opposite side of the chamber and equidistant from each hole. Each chamber was automatically controlled by Packwin software (Panlab S.P., Spain).
As for the cocaine operant self-administration experiment, rats were divided into three different experimental groups: ‘SA pellets’, ‘Yoked pellets’ and ‘Yoked control’. ‘SA pellets’ and ‘Yoked pellets’ rats were initially given access to food pellets in their home cage (10 pellets per day during 5 consecutive days) to get used to the reinforcer. In a first training phase, rats from each group were placed in the chamber for 15 min and the panel of the magazine in open position. During this phase, for the ‘SA pellets’ and the ‘Yoked pellets’ groups, the magazine was filled with 15 food pellets to familiarize rats to eat the reinforcer, but it remained empty for the ‘Yoked control’ rats. In a second training phase, rats received two food magazine training sessions (20 min/session, 1 session/d), in which 20 food pellets were delivered according to a variable time schedule (mean = 60 s) for the ‘SA pellets’ and the ‘Yoked pellets’ groups. No pellet was delivered for the animals of the ‘Yoked control’ group. The house-light was turned off during this phase. On the first session, the magazine was blocked in order to maintain the food magazine open. For the second session, as for the sessions of the food pellets operant self-administration procedure, rats had to push the panel to retrieve the food pellet.
A procedure similar to that used for the cocaine operant self-administration experiment was carried out. ‘SA pellets’ rats were first submitted to a FR1 schedule of reinforcement during daily 2 h sessions for 4 d, and then to a FR5 schedule during daily 2 h sessions for 6 d. When the required number of NPs into the active hole was reached, a food pellet was delivered. NPs into the inactive hole had no programmed consequence. No cutoff was applied concerning the number of food pellets delivered the rat was able to induce during each session. Two yoked groups were exposed to the same associated cues based on the ‘SA pellets’ group. Each rat from these groups was paired with a rat from the ‘SA pellets’ group. Rats from the ‘Yoked pellets’ group received passively the same amount of food pellets as that of ‘SA pellets’ paired rats, whereas no food pellet was delivered to rats from the ‘Yoked control’ group. Rats subjected to cocaine and food self-administration experiments were randomly distributed for mRNA and immunohistochemistry analyses, based on their homogeneous score in respect to self-administration.
Reverse transcription-quantitative PCR analysis
RNA extraction, cDNA synthesis and real-time polymerase chain reaction (PCR) were performed as previously described (Ludwig et al., 2000; Carouge et al., 2010). Primers were from Sigma-Aldrich Co. and are listed in Table 1. For the detailed protocol of release experiments, see Supplement 2 (available online).
Primer sequences
| Gene . | Primer sequence (5′−3′) . | Primer names . | GenBank Acc. # . |
|---|---|---|---|
| PP1β | TGGTGGTATGATGAGTGTGGA | FA | BC062033.1 |
| ACCTTTTCTTCGGTGGATTAG | RB2 | ||
| HDAC2 | CCCTCAAACATGACAAACCA | F | NM_053447.1 |
| TGTCAGGGTCTTCTCCATCC | R | ||
| Mecp2 | AAGTCTGGTCGCTCTGCTG | FP | DQ897369.1 |
| TCTCCCAGTTACAGTGAAGTC | RQ | ||
| 36B4 | GTGCCTCACTCCATCATCAA | FA | NM_022402 |
| TCCGACTCTTCCTTTGCTTC | RB | ||
| Reelin | GCAATCCATCCTTCCACCTC | RLN E57F | NM_080394 |
| GCTTCACAACCCACCACAA | RLN E58R | ||
| GAD67 | CAAGTTCTGGCTGATGTGGA | GAD67F | GQ468528 |
| TCGGAGGCTTTGTGGTATGT | GAD67R |
| Gene . | Primer sequence (5′−3′) . | Primer names . | GenBank Acc. # . |
|---|---|---|---|
| PP1β | TGGTGGTATGATGAGTGTGGA | FA | BC062033.1 |
| ACCTTTTCTTCGGTGGATTAG | RB2 | ||
| HDAC2 | CCCTCAAACATGACAAACCA | F | NM_053447.1 |
| TGTCAGGGTCTTCTCCATCC | R | ||
| Mecp2 | AAGTCTGGTCGCTCTGCTG | FP | DQ897369.1 |
| TCTCCCAGTTACAGTGAAGTC | RQ | ||
| 36B4 | GTGCCTCACTCCATCATCAA | FA | NM_022402 |
| TCCGACTCTTCCTTTGCTTC | RB | ||
| Reelin | GCAATCCATCCTTCCACCTC | RLN E57F | NM_080394 |
| GCTTCACAACCCACCACAA | RLN E58R | ||
| GAD67 | CAAGTTCTGGCTGATGTGGA | GAD67F | GQ468528 |
| TCGGAGGCTTTGTGGTATGT | GAD67R |
Primer sequences used for real-time qPCR are indicated with gene names and GenBank/NCBI accession numbers. F and R indicate forward and reverse primers, respectively.
Primer sequences
| Gene . | Primer sequence (5′−3′) . | Primer names . | GenBank Acc. # . |
|---|---|---|---|
| PP1β | TGGTGGTATGATGAGTGTGGA | FA | BC062033.1 |
| ACCTTTTCTTCGGTGGATTAG | RB2 | ||
| HDAC2 | CCCTCAAACATGACAAACCA | F | NM_053447.1 |
| TGTCAGGGTCTTCTCCATCC | R | ||
| Mecp2 | AAGTCTGGTCGCTCTGCTG | FP | DQ897369.1 |
| TCTCCCAGTTACAGTGAAGTC | RQ | ||
| 36B4 | GTGCCTCACTCCATCATCAA | FA | NM_022402 |
| TCCGACTCTTCCTTTGCTTC | RB | ||
| Reelin | GCAATCCATCCTTCCACCTC | RLN E57F | NM_080394 |
| GCTTCACAACCCACCACAA | RLN E58R | ||
| GAD67 | CAAGTTCTGGCTGATGTGGA | GAD67F | GQ468528 |
| TCGGAGGCTTTGTGGTATGT | GAD67R |
| Gene . | Primer sequence (5′−3′) . | Primer names . | GenBank Acc. # . |
|---|---|---|---|
| PP1β | TGGTGGTATGATGAGTGTGGA | FA | BC062033.1 |
| ACCTTTTCTTCGGTGGATTAG | RB2 | ||
| HDAC2 | CCCTCAAACATGACAAACCA | F | NM_053447.1 |
| TGTCAGGGTCTTCTCCATCC | R | ||
| Mecp2 | AAGTCTGGTCGCTCTGCTG | FP | DQ897369.1 |
| TCTCCCAGTTACAGTGAAGTC | RQ | ||
| 36B4 | GTGCCTCACTCCATCATCAA | FA | NM_022402 |
| TCCGACTCTTCCTTTGCTTC | RB | ||
| Reelin | GCAATCCATCCTTCCACCTC | RLN E57F | NM_080394 |
| GCTTCACAACCCACCACAA | RLN E58R | ||
| GAD67 | CAAGTTCTGGCTGATGTGGA | GAD67F | GQ468528 |
| TCGGAGGCTTTGTGGTATGT | GAD67R |
Primer sequences used for real-time qPCR are indicated with gene names and GenBank/NCBI accession numbers. F and R indicate forward and reverse primers, respectively.
Immunohistochemistry
Immunostaining for Mecp2 and PP1Cβ was performed using standard protocols described earlier (Host et al., 2011; Pol Bodetto et al., 2013). For the detailed protocol of immunohistochemistry, see Supplement 3 (available online).
Statistical analysis
In behavioral experiments, the preference for the active vs. inactive hole was evaluated by comparing the percentage of NPs performed into the active hole (%NPa) to 50% considered as random. Daily and mean performances realized during the FR1 and FR5 periods were compared to 50%. The task acquisition was evaluated by comparing the %NPa to 80%, considered as the acquisition criterion. Daily and mean performances during both FR1 and FR5 periods) were compared to 80%. The number of NPs into the active (NPa) and the inactive hole (NPi) was evaluated with two-way analysis of variance (ANOVA). Reward self-delivery was assessed by one-way ANOVA with repeated measures in order to evaluate ‘Day’ effect. ‘FR’ effect was assessed by one-way ANOVA. In RT-qPCR and immunohistochemistry analyses, one-way ANOVA was performed to evaluate ‘Group’ effect. Newman–Keuls post-hoc was performed, if required. Significance was set at p ⩽ 0.05. Data are expressed as means ± s.e.m.
Results
Acquisition of the operant conditioning task by cocaine or food pellets self-administering rats
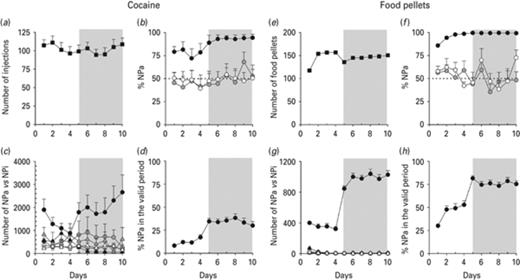
For cocaine or food pellets self-administration, rats were submitted to a FR1 schedule of reinforcement for 4 d, and then to a FR5 schedule of reinforcement for 6 d. They self-administered about 110 cocaine injections per day during the whole experiment (Fig. 1a) and no significant changes were observed from one session to another whatsoever the FR schedule considered (FR1 vs. FR5). Similarly, rats self-delivered about 150 food pellets per day (Fig. 1e), except for the first day of each schedule of reinforcement, and this number was not altered by the FR schedule. The performance came from the rapid preference for the active hole, as the percentage of NP in the active hole (NPa) associated with cocaine (Fig. 1b) or food (Fig. 1f) self-delivery was above 80%, which is highly different from 50%. In cocaine self-administering rats, the acquisition criterion of 80% was achieved after the switch from FR1 to FR5 schedule of reinforcement at day 6 (Fig. 1b). In rats self-delivering food pellets, it was already observed during the FR1 schedule (Fig. 1f). In contrast, rats from all yoked groups (Fig. 1b, f) displayed no preference for the active or the inactive hole in none of the sessions, as the percentage of NPa did not significantly differ from 50%. Accordingly, no significant difference in the number of NPa and NPi performed by yoked rats was noticed (Fig. 1c, g). Surprisingly, yoked rats from the food pellets experiment made almost no NPs at all (Fig. 1g), as compared to yoked rats from the cocaine experiment (Fig. 1c). This discrepancy may be attributed to the nature of the reinforcer either administered or delivered, or from environmental factors such as enclosure background. Contrary to yoked animals, rats self-administering cocaine made much more NPa than NPi especially when submitted to the FR5 schedule (Fig. 1c), as those performing food operant conditioning during both schedules of reinforcement (Fig. 1g). Taken together, the data show that only self-administering rats differentiate the hole associated with cocaine or food pellets delivery from the inactive hole, and therefore fulfill behavioral acquisition rules. Rats self-administering cocaine (Fig. 1d) made much more NPa during the time-out period without any reinforcement than those self-delivering food pellets (Fig. 1h) during both FR schedules of reinforcement. Indeed, at the end of the experiment, only 30% NPa of rats self-administering cocaine were made during the valid period, whereas about 75% NPa of rats self-delivering food pellets were made during the valid period.
Cocaine (a–d) and food pellets (e–h) operant self-administration. The white and gray areas materialize the FR1 and the FR5 schedules of reinforcement, respectively. Number of cocaine injections self-administered by ‘SA cocaine’ rats (a) and number of food pellets self-delivered by ‘SA pellets’ rats (e). (b, f) Percentage of nose pokes (NPs) in the active hole (NPa). Chance level is represented by a dotted line at 50% NPa. (c, g) Number of NPs in the active hole (NPa) and in the inactive hole (NPi) is represented by circles and triangles, respectively. (d, h) Percentage of NPs in the active hole (NPa) performed during the valid period. Circles and triangles refer to NPa and NPi, respectively. Black circles and triangles represent rats self-administering cocaine or self-delivering food pellets, while gray ones represent yoked rats receiving passively the same amount of cocaine or food pellets. White circles and triangles correspond to yoked rats receiving either NaCl instead of cocaine or no food pellets. Data represent the mean ± s.e.m., n = 8–10 per group for the cocaine experiment (ABCD) and n = 8–12 per group for the food pellets one (EFGH).
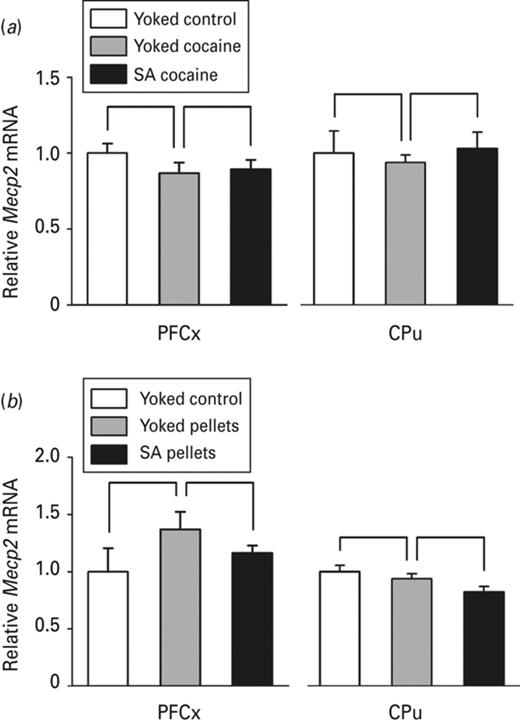
Passive and voluntary cocaine intake similarly regulate Mecp2 expression in brain structures of the reward system
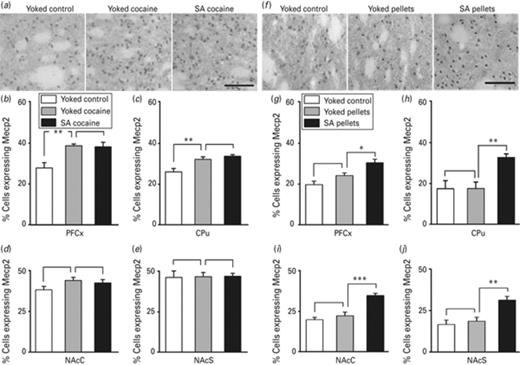
To evaluate the effect of cocaine and its associated cues on Mecp2 expression, we first compared rats receiving passive cocaine injections to rats receiving passive saline injections. Cocaine operant conditioning effect was also addressed by comparing rats receiving passive cocaine injections to rats self-administering cocaine. Mecp2 expression was investigated by RT-qPCR (Fig. 2a) and by immunohistochemistry (Fig. 3a–e) 24 h after the beginning of the last session of cocaine self-administration. No difference was observed in Mecp2 mRNA levels in none of the experimental groups neither in the PFCx, nor in the CPu (Fig. 2a). However, the number of Mecp2 positive cells was increased in these two brain structures in response to passive cocaine injection, as illustrated in the PFCx (Fig. 3b) and in the CPu (Fig. 3c), but not in the NAc subregions (Fig. 3d). There was no difference in the number of Mecp2 immunoreactive cells between rats receiving cocaine passively and self-administering rats, in none of the brain structures analyzed (Fig. 3b–e). Our data are consistent with the induction of Mecp2 protein by cocaine reported in the dorsal striatum and not in the NAc 24 h after the last session of self-administration (Cassel et al., 2006; Im et al., 2010; Host et al., 2011) and highlight the effect of cocaine reward together with its associated cues, rather than that of the operant conditioning associated with drug self-administration.
Methyl-CpG binding protein 2 (Mecp2) mRNA expression in the prefrontal cortex (PFCx) and the caudate putamen (CPu) in response to passive vs. voluntary cocaine or food pellets delivery. The effects of cocaine or food reward and that of operant conditioning on Mecp2 mRNA level were evaluated by quantitative RT-PCR 24 h after the beginning of the last session of cocaine (a) or food pellets administration (b) in the medial part of the PFCx and in the CPu, as indicated. The effect of passive cocaine or food pellets intake was investigated by comparing either ‘Yoked cocaine’ (n = 5) to ‘Yoked control’ rats (n = 4) (a) or ‘Yoked pellets’ (n = 6) to ‘Yoked control’ rats (n = 4) (b), whereas the effect of voluntary intake was evaluated by comparing either ‘SA cocaine’ (n = 5) to ‘Yoked cocaine’ rats (a) or ‘SA pellets’ (n = 5) to ‘Yoked pellets’ rats (b). The amount of Mecp2 transcript was normalized to that of 36B4 mRNA. Data represent the mean ± s.e.m. Statistical analysis performed was one-way analysis of variance (ANOVA).
Methyl-CpG binding protein 2 (Mecp2) protein expression in major brain structures of the reward system of rats self-administering cocaine or self-delivering food pellets. The expression of Mecp2 protein was evaluated by immunohistochemistry in brain structures of rats self-administering cocaine (a–e) or self-delivering food pellets (f–j) 24 h after the beginning of the last session of administration. Representative Mecp2 immunostaining performed in the caudate putamen (CPu) is shown for rats self-administering cocaine (A) and rats self-delivering food pellets (f). Scale bar applicable to all micrographs, 100 μm. Quantification of Mecp2 immunoreactivity was carried in the PFCx (b, g), the CPu (c, h), the nucleus accumbens core (NAcC) (d, i) and the nucleus accumbens shell (NAcS) (e, j). Data represent the mean ± s.e.m., Yoked control, n = 4; Yoked cocaine, n = 5; SA cocaine, n = 5 (b–e), Yoked control, n = 4; Yoked pellet, n = 6, SA pellet, n = 6 (g–j). Statistical analysis performed was one-way analysis of variance (ANOVA) followed by Newman–Keuls post-hoc, when required. *p < 0.05, **p < 0.01, ***p < 0.001. PCFx: prefrontal cortex.
Enhanced Mecp2 expression by food in reward-related brain structures requires learning and memory processes involved in operant conditioning
Mecp2 mRNA expression was next investigated in response to food pellets administration in the same brain structures. The expression was not affected by food intake when evaluated 24 h after the last session in none of the experimental groups (Fig. 2b) neither in the PFCx, nor in the CPu. In contrast, Mecp2 protein expression showed a quite different pattern. Although the number of Mecp2 positive cells was increased by both passive and voluntary cocaine intake, this number evaluated following passive food pellets delivery did not differ from that observed in control rats receiving no food in all structures examined (Fig. 3g–j). Unlike cocaine, food reward by itself was therefore not sufficient to alter Mecp2 expression. However, the number of Mecp2 immunoreactive cells was increased in food pellets self-delivering rats compared to rats receiving food pellets passively, not only in the PFCx (Fig. 3g) and the CPu (Fig. 3h), but also in the NAcC (Fig. 3i) and the NAcS (Fig. 3j). Thus, unlike cocaine reward and its associated cues that were sufficient to induce Mecp2 expression, learning and memory mechanisms involved in operant conditioning are required to induce Mecp2 expression when a natural reward is used.
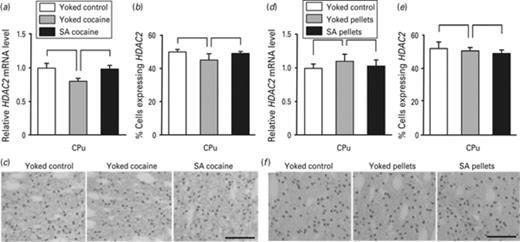
HDAC2 expression is not altered by cocaine or food self-administration
As Mecp2 interacts with HDAC2 in a complex inducing chromatin remodeling and transcriptional repression, we evaluated HDAC2 expression by RT-qPCR and by immunohistochemistry. In the CPu, the amount of HDAC2 transcript was not affected neither by cocaine (Fig. 4a), nor by food intake (Fig. 4d) and the number of immunoreactive cells and their staining intensity remained unaffected by cocaine (Fig. 4b) and by food intake (Fig. 4e, f). Similar observations were made in the PFCx and the NAc (Supplemental Figs 1 and 2), although cocaine was previously shown to induce HDAC2 expression in various brain structures including the CPu, when evaluated after a shorter time following the last cocaine administration (Cassel et al., 2006; Host et al., 2011). Taken together, the data suggest a transient dynamic HDAC2 regulation in response to both passive and voluntary cocaine intake.
HDAC2 expression in the caudate putamen in response to passive vs. voluntary cocaine or food pellets delivery. The effect of cocaine (a–c) and food pellets (d–f) on HDAC2 expression was evaluated in the CPu 24 h after the beginning of the last session of administration by quantitative RT-PCR (a, d) and by immunohistochemistry (b, c, e, f). The amount of HDAC2 transcript was normalized to that of 36B4 mRNA. For ‘Yoked control’, ‘Yoked cocaine’ and ‘SA cocaine’ rats, data represent the mean±s.e.m., n = 4–5 per group (AB), and for ‘Yoked control’, ‘Yoked pellets’ and ‘SA pellets’ rats, data represent the mean ± s.e.m., n = 4–6 per group (DE). Statistical analysis performed was one-way ANOVA. Immunohistochemistry of HDAC2 in the CPu (c, f), scale bar applicable to all micrographs, 100 μm.
PP1Cβ repression by passive and voluntary cocaine administration correlates with Mecp2 expression in specific brain structures of the reward system
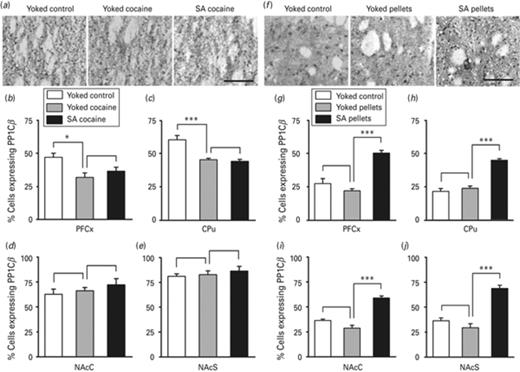
We next checked whether enhanced levels of Mecp2 observed in the PFCx and the CPu in response to cocaine intake translated into decreased expression of PP1Cβ. PP1Cβ mRNA level was found to be strongly repressed in both the PFCx (Fig. 5a) and the CPu (Fig. 5b) of rats receiving cocaine compared to saline-treated rats. No difference in PP1Cβ mRNA level was observed in rats receiving cocaine voluntarily or passively neither in the PFCx, nor in the CPu (Fig. 5a, b). We then verified that protein expression reflected the corresponding mRNA expression. A decrease in the number of PP1Cβ immunoreactive cells was observed in the PFCx (Fig. 6b) and the CPu (Fig. 6c) of rats receiving cocaine, compared to rats treated with the vehicle. As for Mecp2 (Fig. 3d, e), no difference was noticed in PP1Cβ protein expression in the NAc subregions, neither in the NAcC, nor in the NAcS of these two groups of rats (Fig. 6d). As for PP1Cβ mRNA level, there was no effect of cocaine operant conditioning, as the number of PP1Cβ immunoreactive cells did not differ between the two groups of rats receiving cocaine, in none of the brain structures evaluated (Fig. 6b–e). The data therefore underline, as for Mecp2, the effect of cocaine reward and its associated cues on PP1Cβ expression and not that of cocaine operant conditioning. They also reveal an inverse correlation between the levels of the two proteins in the PFCx and the CPu consistent with the role of Mecp2 acting as a repressor of PP1β (Pol Bodetto et al., 2013).
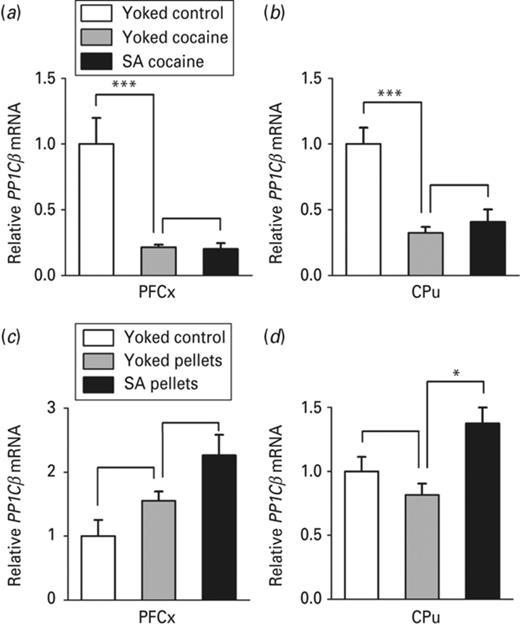
PP1Cβ mRNA expression in the pre-frontal cortex (PFCx) and the caudate putamen (CPu) in response to passive vs. voluntary cocaine or food pellets delivery. The effects of cocaine and food reward and that of related operant conditioning on PP1Cβ mRNA level were evaluated by quantitative RT-PCR 24 h after the beginning of the last session of cocaine (a, b) and food pellets (c, d) administration in the medial part of the PFCx and in the CPu, as indicated. The amount of PP1Cβ transcript was normalized to that of 36B4 mRNA. Data represent the mean ± s.e.m., n = 4–6 per group (ABCD). Statistical analysis performed was one-way analysis of variance (ANOVA) followed by Newman–Keuls post-hoc, when required. *p < 0.05, ***p < 0.001.
PP1Cβ protein expression in major brain structures of the reward system of rats self-administering cocaine or self-delivering food pellets PP1Cβ protein expression was evaluated by immunohistochemistry 24 h after the beginning of the last session of cocaine (a–e) or food pellets (f–j) administration. Representative PP1Cβ immunostaining performed in the caudate putamen (CPu) is shown for rats self-administering cocaine (a) and rats self-delivering food pellets (f) together with control yoked experiments, as indicated. Scale bar of 100 μm is applicable to all micrographs. Quantification of PP1Cβ immunoreactivity was carried in pre-frontal cortex (PFCx) (b, g), the CPu (c, h), the nucleus accumbens core (NAcC) (d, i) and the nucleus accumbens shell (NAcS) (e, j), as indicated. Data represent the mean ± s.e.m., n = 4–5 per group (b–e), n = 4–6 per group (g–j). Statistical analysis performed was one-way analysis of variance (ANOVA) followed by Newman–Keuls post-hoc, when required. *p < 0.05, ***p < 0.001.
PP1Cβ is only induced by voluntary food delivery in the PFCx, the CPu and the NAc
We next investigated whether PP1Cβ expression could be differentially regulated by yoked food pellets delivery vs. food pellets delivered voluntarily. PP1Cβ mRNA level was induced in the CPu of food pellets self-delivering rats compared to rats with passive food delivery (Fig. 5d). A similar pattern was observed in the PFCx, even if significance was not reached (Fig. 5c). Contrary to cocaine self-administration experiments, we did not observe any difference in PP1Cβ mRNA level between the two yoked groups, neither in the PFCx, nor in the CPu, which shows that PP1Cβ expression is not regulated by natural reward alone (Fig. 5a, bvs.Fig. 5c, d). When measuring protein expression, we found an increase in the number of PP1Cβ immunoreactive cells in the PFCx (Fig. 6g), the CPu (Fig. 6h), the NAcC (Fig. 6i) and the NAcS (Fig. 6j) of food pellets self-delivering rats compared to rats receiving food pellets passively. In agreement with the expression of PP1Cβ mRNA, food pellets reward alone did not alter the number of PP1Cβ immunoreactive cells in none of the brain structures evaluated, as their number did not differ between rats receiving food pellets passively and rats receiving no food pellet. Thus, although cocaine reward and its associated cues are sufficient to repress PP1Cβ, food reward alone does not affect its expression that is only induced upon food operant conditioning. Such an opposite regulation by cocaine and food underlines a striking different involvement of PP1Cβ in mediating the response to the two rewarding stimuli.
Reelin and GAD67 genes are differentially regulated by cocaine or food
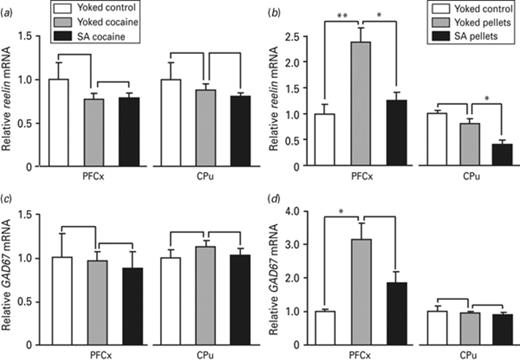
We next checked whether other direct Mecp2 target genes are also differentially regulated by cocaine and food. As Mecp2 is widely expressed in post-mitotic GABAergic neurons in the CPu and the frontal cortex (Cassel et al., 2006), the expression of reelin and glutamic acid decarboxylase 67 (GAD67) genes that are negatively regulated by DNA methylation-dependent mechanisms (Zhubi et al., 2014) was evaluated in these structures. Reelin is preferentially synthesized in GABAergic neurons in the cortex and the CPu (Costa et al., 2009) and GAD67 is a marker for GABAergic neurons (Rasmussen et al., 2007). The expression of both genes was not affected by cocaine, independently of the administration mode (Fig. 7a, c). In contrast, a 2- to 3-fold increase was observed for both genes in response to passive food pellet delivery in the PFCx, but not following contingent delivery (Fig. 7b, d). In the CPu, a 2-fold decrease was observed in reelin expression in rats self-delivering food pellets relative to rats with passive food delivery or yoked control rats (Fig. 7b). GAD67 expression remained unaffected by food pellet delivery (Fig. 7d). Thus, although reelin and GAD67 expression is not affected by cocaine, their regulation by food appears to depend on the brain structure considered and the mode of administration and provides evidence for additional changes in gene expression occurring in response to each reinforcer.
Reelin and GAD67 mRNA expression in the pre-frontal cortex (PFCx) and the caudate putamen (CPu) in response to passive vs. voluntary cocaine or food pellets delivery. The effects of cocaine and food reward and that of related operant conditioning on reelin and GAD67 mRNA level were evaluated by quantitative RT-PCR 24 h after the beginning of the last session of cocaine (a, c) and food pellets (b, d) administration in the medial part of the PFCx and in the CPu, as indicated. The amount of reelin and GAD67 transcripts was normalized to that of 36B4 mRNA. Data represent the mean ± s.e.m., n = 4–6 per group (ABCD). Statistical analysis performed was one-way analysis of variance (ANOVA) followed by Newman–Keuls post-hoc, when required. *p < 0.05, **p < 0.01, ***p < 0.001.
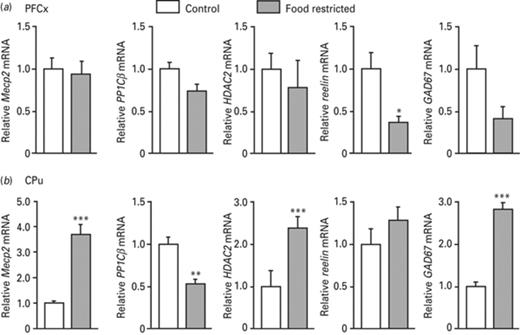
As food restriction was used to motivate rats in operant-conditioning experiments, its effect on gene expression was addressed by comparing yoked control rats from the food pellet experiments that were food restricted, to yoked control rats from the cocaine experiments that were not. Baseline expression of Mecp2, PP1Cβ, HDAC2, reelin and GAD67 genes was simultaneously evaluated in the PFCx and the CPu in both groups. In the PFCx, a 2.5-fold repression was observed for reelin upon food restriction, whereas no significant change was noticed for the other genes (Fig. 8a). In the CPu, except for reelin, the expression of all genes was affected by food restriction (Fig. 8b). A 2.4- to 3.8-fold induction was observed for Mecp2, HDAC2 and GAD67 genes, whereas a 2-fold repression was observed for PP1Cβ. That Mecp2 was increased by food restriction may reflect a reward for food that has been reported in some Mecp2 mutant mice or in human males with hypomorphic mutations overexpressing the gene or in females with milder variants of Rett syndrome that often become obese (Fyffe et al., 2008). HDAC2 induction by food restriction appears consistent with enhanced HDAC2 labeling intensity observed in food-restricted control animals (Fig 4c, f). This induction in the CPu suggests that it could participate to the 2-fold repression of PP1Cβ resulting from food restriction (Fig. 8b), consistent with a decrease in PP1Cβ immunoreative cells in control food-restricted rats (Fig. 6f, h) relative to rats that were not food restricted (Fig. 6a, c).
Comparison of Mecp2, PP1Cβ, HDAC2, reelin and GAD67 gene expression in the pre-frontal cortex (PFCx) and the cauate putamen (CPu) in response to food restriction. Yoked control rats from the cocaine experiment that are not food restricted were compared to those from the food pellet experiment that are food restricted. Expression of the five genes was measured in the PFCx (a) and the CPu (b) by quantitative RT-PCR and normalized to the expression of 364B. Data represent the mean ± s.e.m. of the control group (n = 4) and that of the food-restricted rats (n = 4). Statistical analysis performed was one-way analysis of variance (ANOVA). *p < 0.05, **p < 0.01, ***p < 0.001.
Discussion
In the present report, we examined the effect of passive vs. voluntary cocaine intake on Mecp2 and PP1Cβ expression. Cocaine-induced repression of PP1Cβ in the PFCx and the CPu was inversely correlated with Mecp2 levels and was similar, independently of the administration mode, further supporting the role of Mecp2 as a repressor of PP1Cβ. Accordingly, no change in PP1Cβ expression was observed in the NAc in which Mecp2 levels remained unaffected by cocaine administration. When comparing cocaine effects to those of food, a striking different expression pattern was observed for both proteins. Despite food rewarding effects, their levels remained unaffected by passive delivery in all brain structures examined, as both proteins were induced by food operant conditioning in the PFCx and throughout the striatum. Their regulation differed depending the brain structures considered, the nature of the reinforcer and operant conditioning-related memory processes. As the mesostriatal dopamine system is activated by food and by drugs of abuse, the data provide new insights into the mechanism by which two proteins involved in cocaine reward (Zachariou et al., 2002; Romieu et al., 2008; Feng and Nestler, 2010), or in food consumption (Fyffe et al., 2008; Danielli et al., 2010; Cui et al., 2012) are differentially regulated.
Food restriction induced significant changes in the baseline gene expression of Mecp2, PP1Cβ, HDAC2 and GAD67 in the CPu (Fig. 8b), indicating that the comparison between rats exposed to cocaine administration vs. food delivery in this brain structure has to be taken with caution, notably in respect to the regulation of PP1Cβ by Mecp2. In contrast, food restriction did not affect the basal expression of most genes in the PFCx (Fig. 8a) and therefore allows a comparison between their relative expressions in response to cocaine vs. food. Mecp2 and PP1Cβ proteins being differentially regulated by cocaine and food in the CPu and the PFCx, their regulation in these structures is likely to result from different molecular mechanisms.
Our data comfort the cocaine-induced increase in Mecp2 protein expression previously reported (Cassel et al., 2006; Im et al., 2010; Host et al., 2011). However, this increase observed 24 h after the last self-administration session was not accompanied by any changes in Mecp2 mRNA expression. As Mecp2 transcriptional activation was previously observed when evaluated 2 h after the last cocaine self-administration session (Host et al., 2011), such a rapid activation is likely to be transient and whether it could account for a delayed increase in protein expression remains to be determined. Alternatively, cocaine may regulate Mecp2 protein by controlling either positively its translation or negatively its degradation after 24 h without affecting its transcription.
HDAC2 response to cocaine might also be transient, as cocaine-induced HDAC2 mRNA and protein 2 h after the last session of cocaine self-administration (Host et al., 2011), whereas no increase was observed 24 h after the last cocaine intake. The mechanism may contribute to the delayed repression of PP1Cβ, consistent with HDAC2 recruitment by Mecp2 in a complex silencing transcriptional activity (Nan et al., 1998; Harikrishnan et al., 2005). Alternatively, an HDAC-independent mechanism of MeCP2-mediated repression has been reported to be particularly relevant in neurons that have high levels of methylated CpG and MeCP2 (Theisen et al., 2013).
Interestingly, the cocaine-induced Mecp2 expression was in phase with a decrease in PP1Cβ expression independently of the cocaine administration mode. As demonstrated previously, repeated passive cocaine administration repressed PP1Cβ and PP1Cγ expression through DNA hypermethylation and increased Mecp2 binding (Anier et al., 2010; Pol Bodetto et al., 2013). The down-regulation of PP1Cβ observed in cocaine self-administering rats, is therefore likely to result from DNA hypermethylation at the PP1Cβ gene followed by increased Mecp2 binding. Gene regulation by DNA methylation involves MBDs, DNMTs and TET proteins, the two latter controlling the balance between methylated CpG, hydroxymethylated CpG and non-methylated CpG (Guo et al., 2011). Drugs of abuse alter the expression of some MBD and DNMT proteins in the brain (Cassel et al., 2006; Anier et al., 2010; LaPlant et al., 2010; Pol Bodetto et al., 2013), underlining their effects on DNA methylation mechanisms that has been actually reported for few genes. These genes are either repressed by DNA methylation and Mecp2, like CDKL5 (Carouge et al., 2010), PP1Cβ (Pol Bodetto et al., 2013) and PP1Cγ or induced by DNA demethylation like fosB or TACR3 (Anier et al., 2010; Barros et al., 2011). In contrast to PP1Cβ, the expression of reelin and GAD67 genes was not affected by cocaine neither in the PFCx, nor in the CPu, independently of the administration mode (Fig. 7a, c). Although Mecp2 has been shown to bind to them, cocaine may trigger their demethylation, as a crucial role of global PFCx DNA hypomethylation in the rewarding effect of cocaine has been reported (Tian et al., 2012).
As PP1Cβ and γ genes are repressed by cocaine, the overexpression of Mecp2 protein in all brain structures examined (Fig. 3g–j), together with that of PP1Cβ mRNA (Fig. 5c, d) or protein (Fig. 6g–j) in food self-delivering rats was not expected. Similarly, reelin modulation also needs operant conditioning in the CPu, PP1Cβ being induced (Figs 5d and 6h) and reelin being repressed (Fig. 7b), revealing an opposite regulation previously reported in response to contextual fear conditioning (Miller and Sweatt, 2007). Considering the repression of PP1Cβ by food restriction (Fig. 8b), this effect was reversed by contingent food delivery (Figs. 5d and 6h). In contrast, Mecp2 mRNA was induced by food restriction (Fig. 8b) and the percentage of Mecp2 immunoreactive cells was by contingent food delivery (Fig. 3h), observations that appear consistent with the documented role of Mecp2 in reward and memory. On the other hand, most genes were not affected by food restriction in the PFCx except reelin that was repressed (Fig. 8a). That food delivery to food-restricted animals reverses its repression (Fig. 7b) seems to make sense, but that it only occurs in response to passive food delivery is intriguing raising concerns about the mechanism by which a gene usually repressed by Mecp2 and by DNA methylation is induced together with Mecp2. On one hand, Mecp2 may act as a transcriptional repressor or activator depending on its interaction with either a corepressor such as SIN3A, or a coactivator such as CREB (Yasui et al., 2007; Chahrour et al., 2008; Cortes-Mendoza et al., 2013). On the other hand, food, unlike cocaine, may not alter DNA methylation. In line with this hypothesis, DNMT inhibitors (Anier et al., 2010; Han et al., 2010), or Mecp2 repression (Im et al., 2010) or dysfunction (Deng et al., 2010) negatively regulate cocaine or amphetamine intake, whereas administration of DNMT inhibitors does not change sucrose intake (Warnault et al., 2013). These data support the idea that a learning process associated with natural reinforcer may not require de novo DNA methylation that can block access to transcription factors.
In learning and memory processes, the contribution of Mecp2 (Moretti et al., 2006; Pelka et al., 2006; Stearns et al., 2007; Na et al., 2012) and PP1 (Genoux et al., 2002; Miller and Sweatt, 2007; Graff et al., 2010; Koshibu et al., 2011) has been well documented and their control by cocaine may therefore alter these functions. As operant learning processes are involved in self-administration, it is rather surprising that neither Mecp2, nor PP1Cβ protein levels were modified by cocaine operant conditioning compared to passive cocaine injections. However, the effect of cocaine with its associated cues could hide that of operant conditioning. Thus, alteration in Mecp2 and PP1Cβ levels following passive cocaine intake may be sufficient to trigger the rewarding effect of the drug and to contribute to learning and memory processes related to the drug. Despite the rewarding and motivational effects of food resulting from restriction, food reward had no effect on Mecp2 and PP1Cβ protein expression without operant conditioning. Although cocaine operant conditioning did not affect their expression relative to passive cocaine intake, the expression of both proteins was increased in all examined brain structures of food pellets self-delivering rats. These results indicate that the mechanisms underlying operant conditioning differ between drugs of abuse and natural rewards. It therefore supports the concept that addiction may be, at least in part, a learning disorder, consistent with previous reports (Di Chiara, 1999; Hyman, 2005) and raises questions about how gene expression changes occurring in the PFCx and CPu affect learning and memory. Old or remote memory is stored in a cortical network, whereas the striatum has a well-documented role in procedural learning and memory resulting in the formation of stimulus-response habits. In addition, working memory has been associated with striato-frontal brain regions. Changes in gene expression in the PFCx and CPu may therefore be involved in processing these types of memory.
Although Mecp2 mRNA expression was not affected neither by cocaine, nor by food (Fig. 2), Mecp2 protein was induced by both contingent and non-contingent cocaine self-administration (Fig. 3a–c), but only by contingent food delivery in the PFCx and the CPu (Fig. 3f–h). In these structures, expression of PP1Cβ mRNA and protein was repressed by both contingent and non-contingent cocaine administration (Figs 5a, and 6a–c), but was in contrast induced by contingent food delivery (Figs 5c, d and 6f–h). On the other hand, expression of reelin and GAD67 mRNA was altered by food with changes depending on the brain structures and the type of food delivery (Fig. 7b, d), but no change was noticed in response to cocaine (Fig. 7a, c). Such differential regulation of gene or protein expression in response to the two reinforcers was unexpected. Indeed, neural circuits implicated in drug conditioning, craving and relapse extensively overlap with those involved in natural reward and reinforcement like food (Blum et al., 2011). Most of the genes or proteins that have been compared in response to both rewarding agents are similarly regulated. This is the case of ∆fosB and CREB which are induced or activated in striatum following repeated exposure to drugs (McClung and Nestler, 2003) or by natural reward (Shiflett et al., 2009; Christiansen et al., 2011). In addition, ∆fosB overexpression in the NAc or the dorsal striatum enhances food-motivated behavior, as it does for drug rewards (Olausson et al., 2006), whereas manipulation of CREB activity affects drug rewards as well as preference for natural rewards (Barrot et al., 2002). However, studies based on HDAC and DNMT inhibitors provide evidence that drugs of abuse and natural reinforcers do not rely on similar mechanisms, consistent with specific neuroadaptations triggered by each reinforcer (Carelli et al., 2000; Koya et al., 2006; Levy et al., 2007; Chen et al., 2008; DiLeone et al., 2012). Taken together, these studies support the notion that specific hitherto unknown molecular changes in gene expression are induced by each reinforcer activating a common reward circuitry.
In conclusion, passive and voluntary cocaine administration, positively regulate Mecp2 and negatively PP1Cβ protein expression in reward-related brain structures. In contrast, the two proteins were induced by food operant conditioning, no effect being noticed when food was passively delivered. Most genes examined were found to be differentially regulated by cocaine and food depending on the brain structures and the administration mode. Considering the role of these genes in functions such as reward, memory, or gene transcription, their differential regulation by cocaine vs. food provide new insights into the mechanisms underlying the neuroadaptations triggered by each type of reward. Deciphering mechanisms controlling these changes in gene expression triggered by drugs of abuse or natural rewards remains a major issue to understand how they differ in their ability to drive behavior.
Supplementary material
Supplementary material accompanies this paper on the Journal's website.
Acknowledgments
This work was supported by CNRS, by the Université de Strasbourg and by the Association Française du Syndrome de Rett (AFSR). S. Pol Bodetto was a recipient of a fellowship from the ‘Ministère de l'Enseignement Supérieur et de la Recherche’. Authors are grateful to K. Geiger for her technical assistance in transcardial perfusions.
Statement of Interest
The authors declare no conflict of interest.
References
Supplementary Material
Supplementary information supplied by authors.
Supplementary information supplied by authors.
Supplementary information supplied by authors.
Supplementary information supplied by authors.
Supplementary information supplied by authors.
Supplementary information supplied by authors.
Supplementary information supplied by authors.